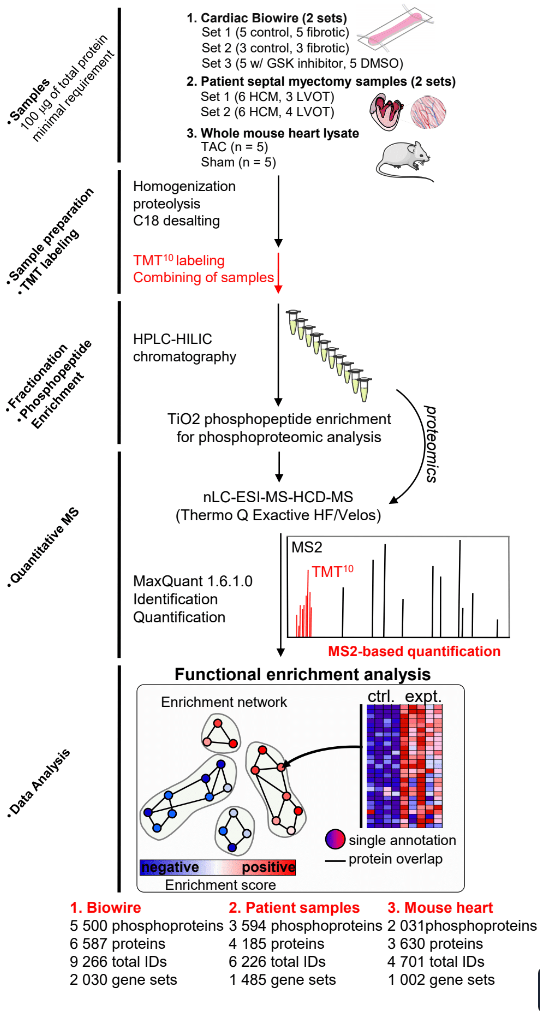
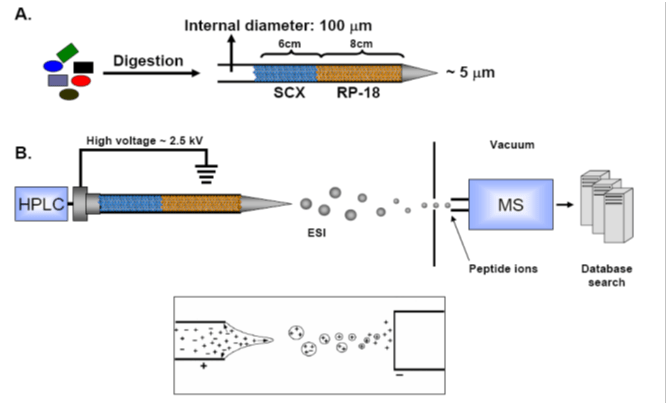
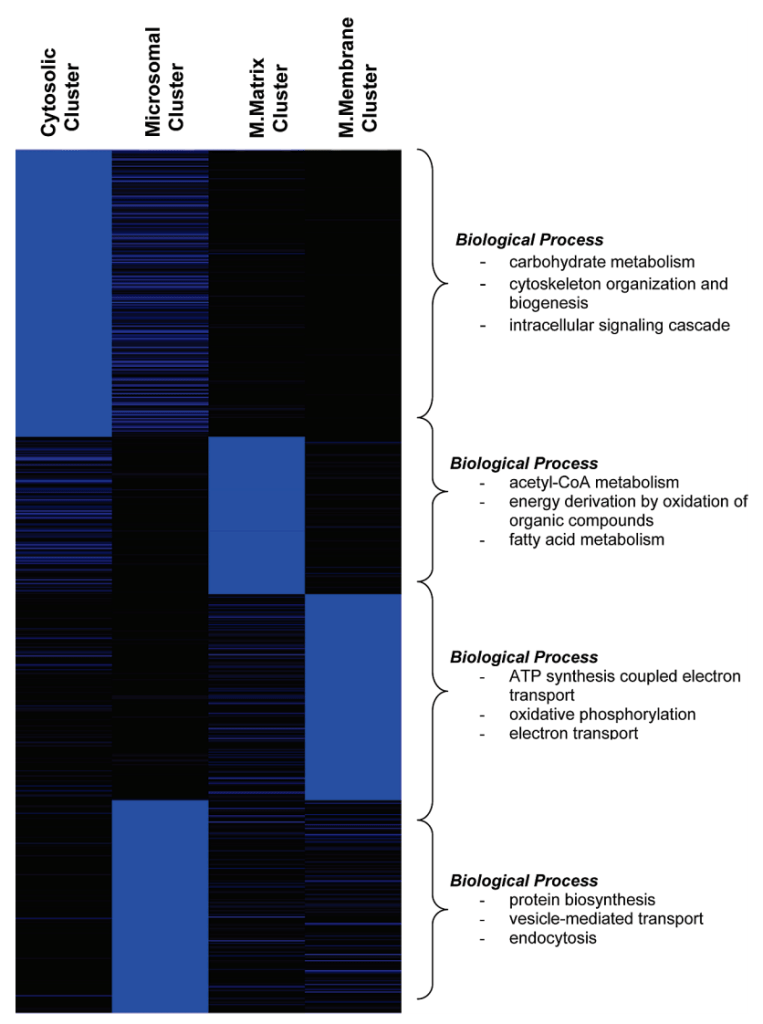
In collaborative studies with proteomic experts Dr. Thomas Kislinger (Ontario Cancer Institute, U of Toronto) and Dr. Andrew Emili (Boston University) we have been developing methodologies for the large scale study of proteomic expression profiles in cardiovascular diseases. We have been applying these techniques to study the mechanisms involved in the progression of heart failure in mouse models, as well as in human patients. These experiments are undertaken with the aim of uncovering new cellular pathways that are involved in the disease in order to develop new therapeutic targets. As well, we have been attempting to identify new blood-borne biomarkers of cardiovascular disease to develop new screening tools.
As we complete our large scale proteomic investigations of mouse and human models of heart failure, we are extending our findings by developing advanced protein-protein interaction (PPI) map of protein networks affected in heart failure. We are in the process of using this information to identify novel cellular targets for development of therapeutic interventions.
Our specific interest and expertise in this project is the application of phenotypic analysis, cellular imaging and protein analyses to identify key proteins that are critical in myocyte biology. We do this by using mammalian cell lines, transgenic and knockout mice, primary cardiac muscle-derived cell cultures, and patient-derived cardiac tissues, to understand cardiac function and disease. We use a combination of molecular biology with computational analyses, where the protein data will be integrated into existing and newly developed bioinformatic tools being maintained within the University of Toronto for detailed analyses and sharing. Our initial focus is the identification of proteins involving several critical calcium regulatory proteins; however, we are extending our abilities rapidly to other key proteins found to be important in heart failure. The emphasis will be on discovering novel protein interactions critical for understanding the progress of cardiomyopathy. The ultimate aim of this project is to provide clinically relevant therapeutic methods that can be applied to the diagnosis and treatment of heart failure in human patients.